Experiencing postpartum depression?
You’re not alone.
If you’re experiencing severe, lasting depression after having a baby, you may have postpartum depression. See if you qualify for this clinical trial with the form below. You could get the chance to try an investigational medication that’s designed to help improve your mental health.

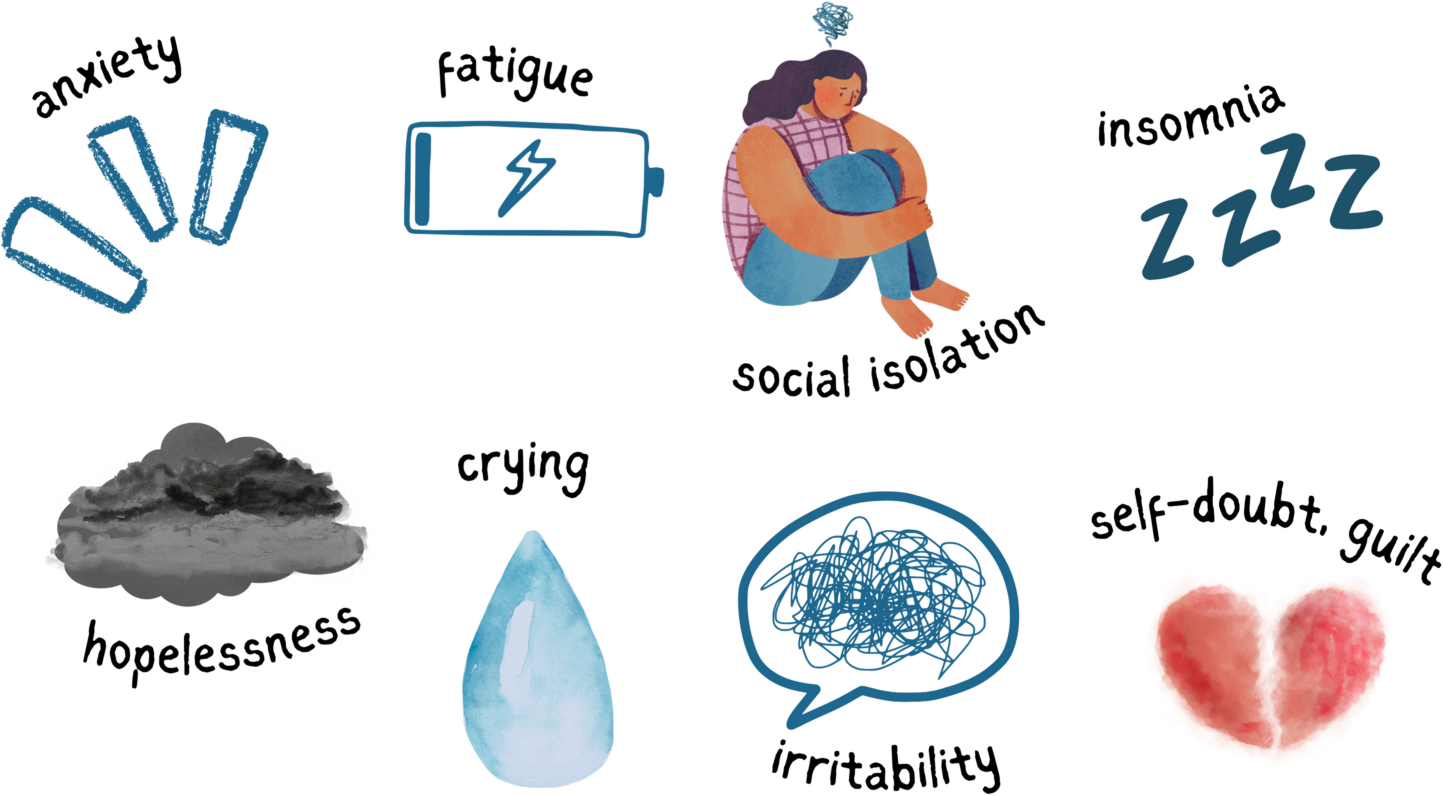
PPD Signs and Symptoms
Sharing is Caring: Does someone you know need to hear about this? Consider sending them this page to show them that help is available.
See if you qualify
Fill out the personal health questions below to see if you meet the basic study requirements. You don’t have to answer any question you don’t want to answer. Your answers will be recorded, but your information will only be used for the purposes of this study.
*Please note that you are also welcome to fill out this form for a loved one who you suspect may be experiencing postpartum depression.
Why participate?
Try an investigational treatment designed to help improve your symptoms
Receive compensation for your time, childcare, formula, and travel
Be a part of helping millions of other mothers with PPD
What’s involved?
For the next 16 days after you get home:
Frequently asked questions
For this study, you’ll need to have a study partner(s) stay with you at home for the 16 days after you get home from your inpatient stay.
Study partners must be 18+, willing and able to receive a short training, and be there for you or the baby just in case. During that time, if you need to leave home, your study partner will need to go with you.
You will also need a backup study partner just in case.
You could use a combination of two different people- for example, have your partner work from home for 10 days, then have a friend work from home and spend the night for the next 6 days. It’s important to us that you find a system that works best for you and your situation, so please ask your study site if you have any questions. The research site will provide more details and logistics information.
